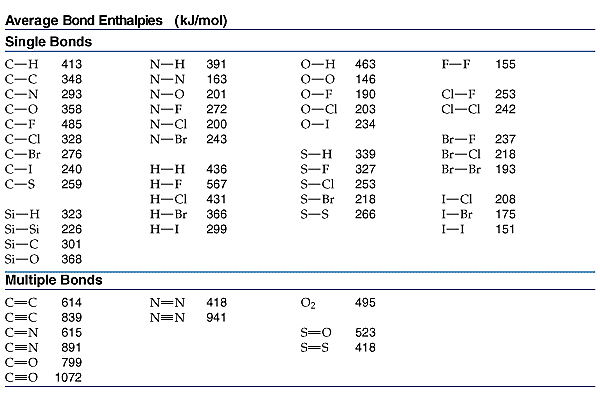
Calculate the enthalpy for the following reaction using the given bond energies (kJ/mol) (C - H = 414;H - O = 463;H - Cl = 431,C - Cl = 326;C - O =
42. 1 mole of 'A', 1.5 mole of 'B' and 2 mole of 'C' are taken in a vessel of volume one litre . At equilibrium concentration of C is 0.5 mole/L.

SOLVED: What is the freezing point, in °C, of a solution made with 1.07 mol of CHCl₃ in 530.0 g of CCl₄ (Kf = 29.8 °C/m, normal freezing point, Tf = -22.9 °C)?
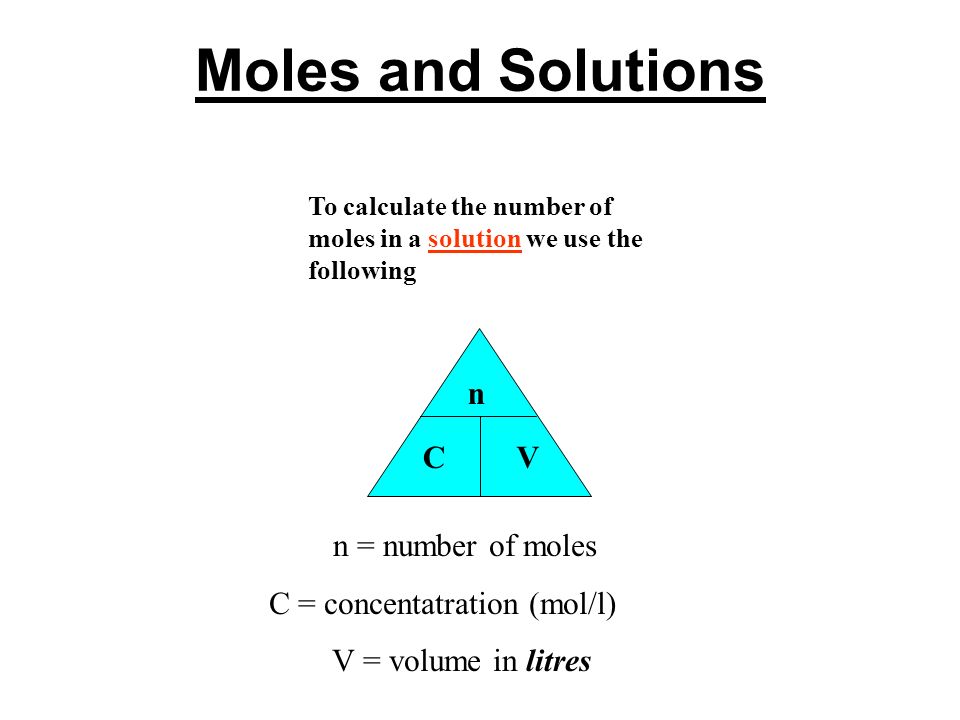
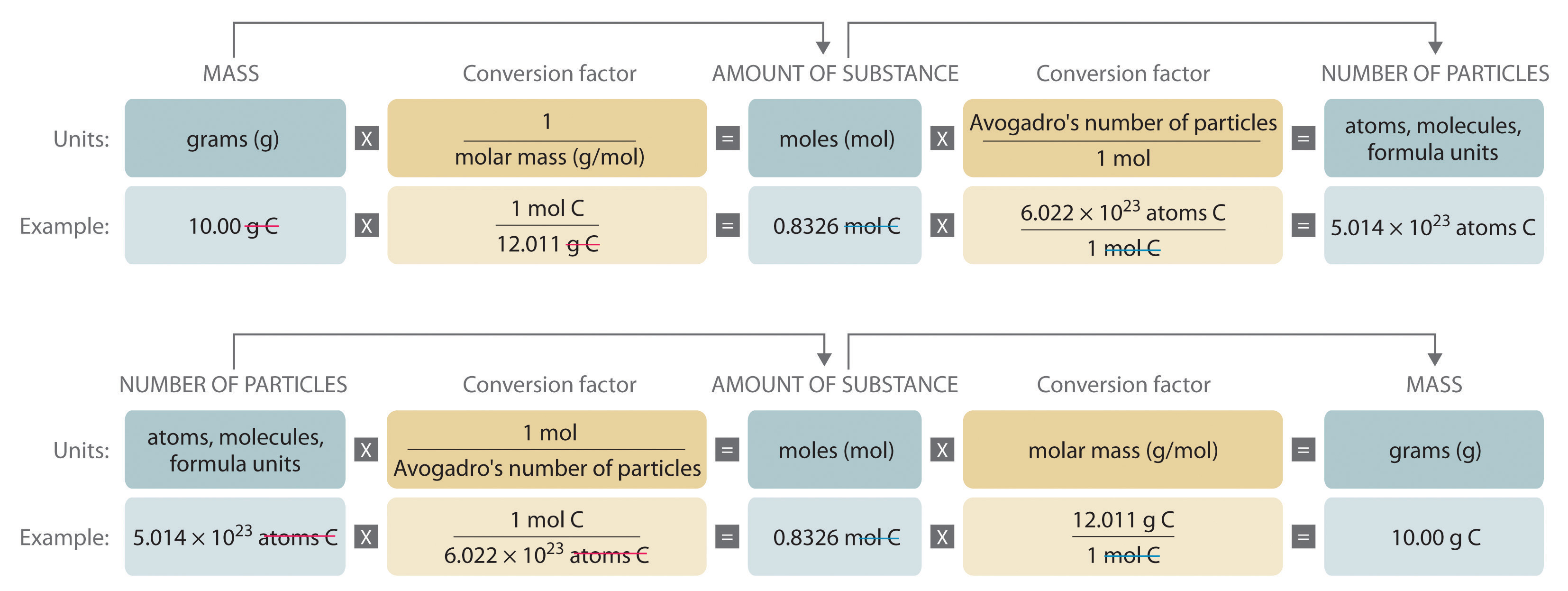
Moles and Solutions g n gfm To calculate the number of moles in a solution we use the following n CV n = number of moles C = concentatration (mol/l) V. - ppt download

Draw the complete molecular orbital diagram for C^+_2 (form the molecular orbital diagram from the combination of a neutral C and a cationic C^+). | Homework.Study.com
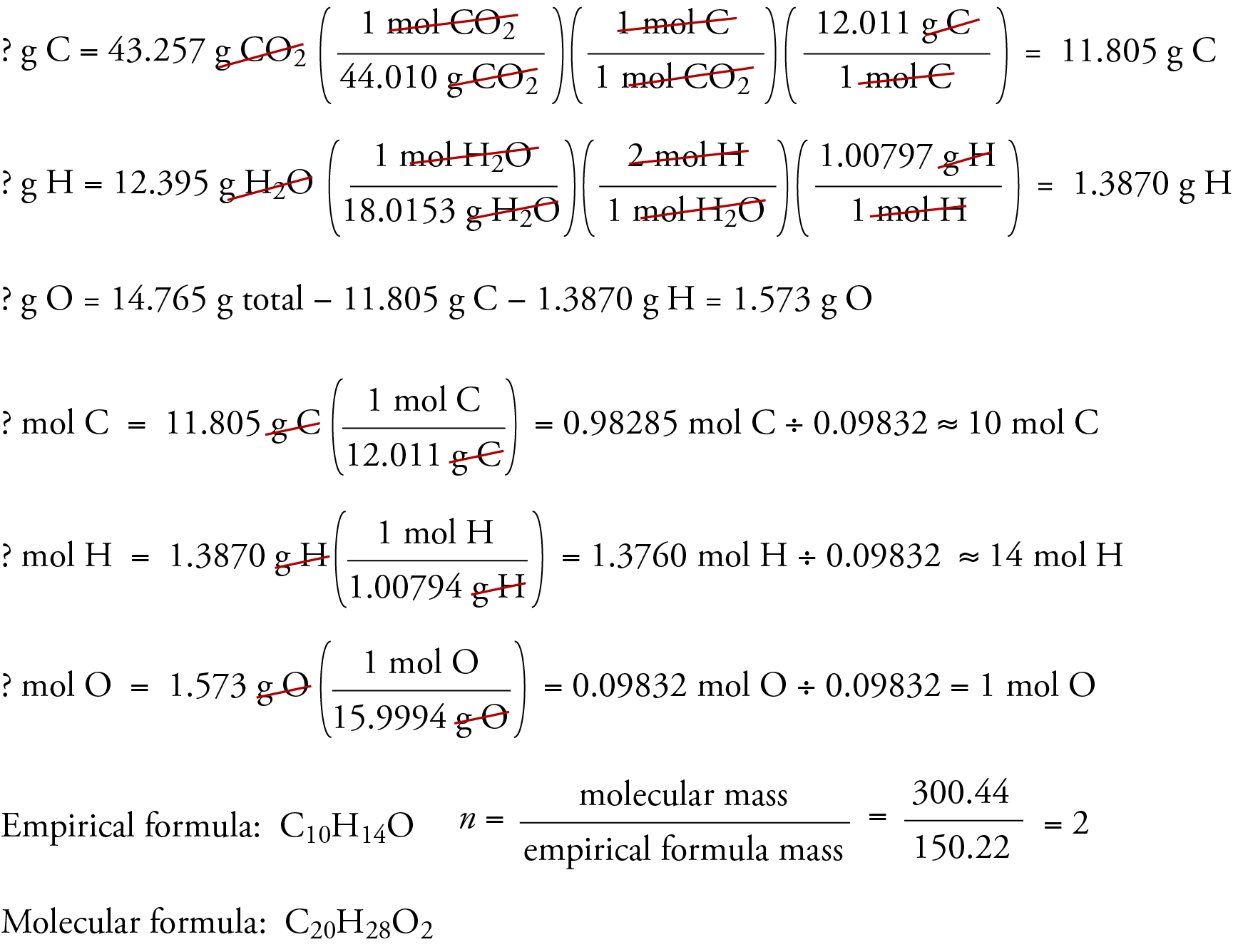
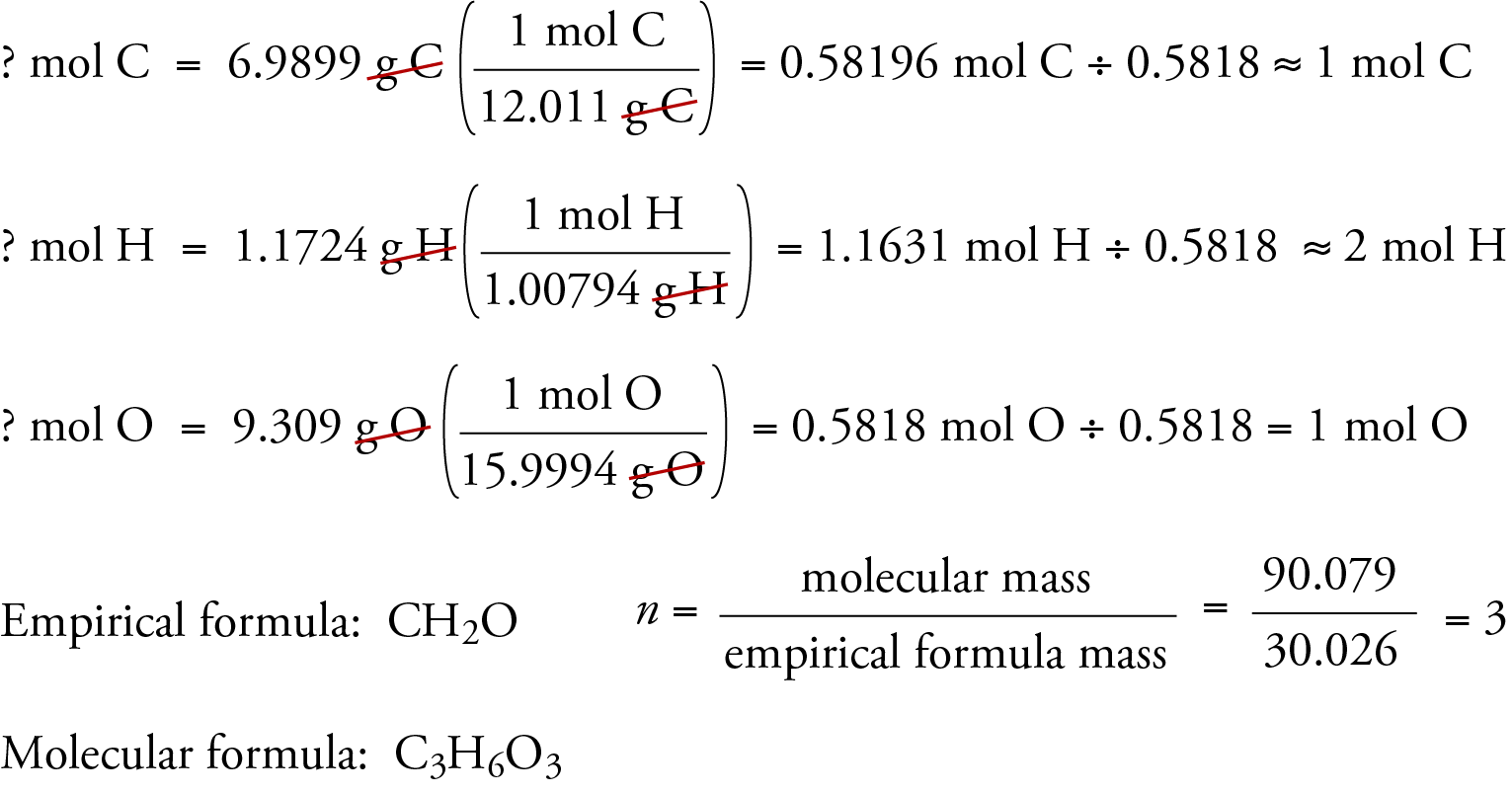
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are
![Molare Masse berechnen • Formel und Berechnung · [mit Video] Molare Masse berechnen • Formel und Berechnung · [mit Video]](https://d1g9li960vagp7.cloudfront.net/wp-content/uploads/2019/10/WP_Molare-Masse_dreieck-1024x576.jpg)