
Chemical Equations And balancing equations. Chemical Equation CH 4 + O 2 CO 2 + H 2 O Reactantsproducts Means to produce. - ppt download

Class11Cac2+H2O=acetylene (calcium carbide react with water)complete reaction explanations in Telugu - YouTube
CaC2 reacts with H2O and gives X. X reacts with Cu2Cl2 in the presence of NH4OH and give Y. What is Y, and how? - Quora

CaC2 + H2O → CaO + HC≡ CH n(CH ≡ CH) + nH2 → - (CH2 - CH2)n - Polyethylene can be produced from calcium carbide according to the given sequence of

How to Balance CaC2+H2O=Ca(OH)2+C2H2|Chemical equation CaC2+H2O =Ca(OH)2+C2H2|CaC2+H2O=Ca(OH)2+C2H2 - YouTube

polyethylene can be produced from CaC2 according to following sequence CaC2+ H2O-CaO + HCtriple bond CH n(HC triple bond CH) - Chemistry - Basic Concepts in Chemistry - 10693899 | Meritnation.com
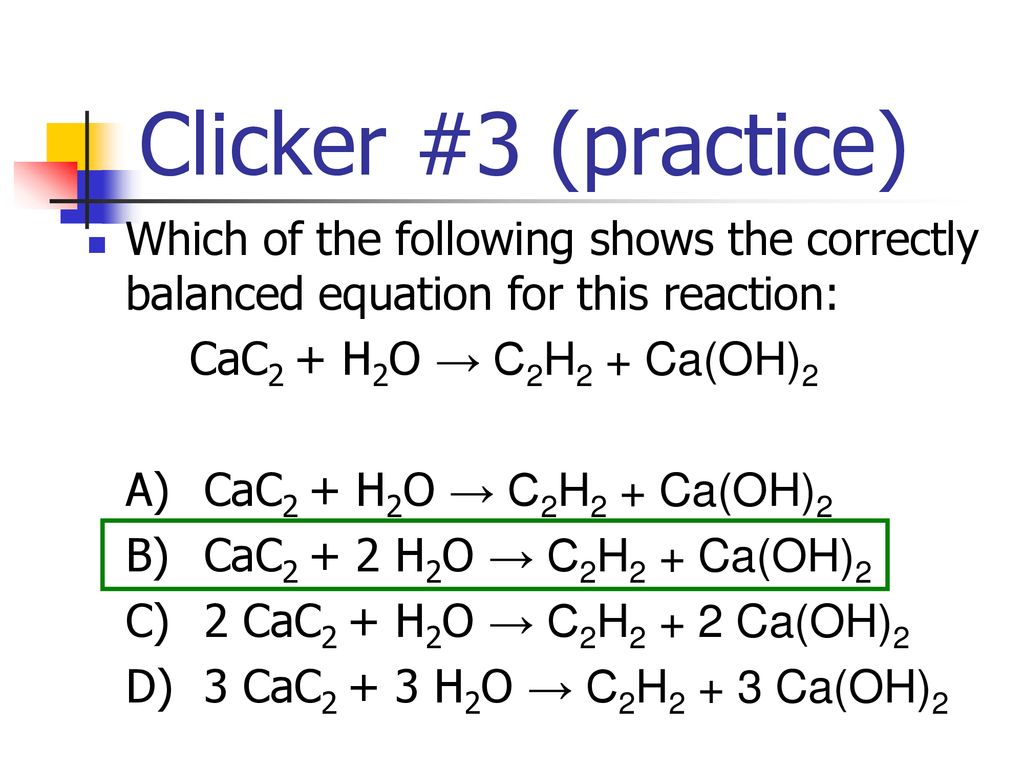
Clicker #1 Which of the following shows the correctly balanced equation for this reaction: H2O2 → H2O + O2 A) 3 H2O2 → 3 H2O + O2 B) H2O2 → 2 H2O + O2. - ppt download
Calcium carbide, CaC2, reacts with water to form ethyne, C2H2, and calcium hydroxide. The equation for the reaction is shown. CaC2(s) + 2H2O(l) → C2H2(g) + Ca(OH) 2(s), which volume of ethyne

Towards C1 chemistry: methanol vinylation by CaC2 in water in the presence of potassium or sodium carbonates - Parshina - 2019 - Journal of Chemical Technology & Biotechnology - Wiley Online Library
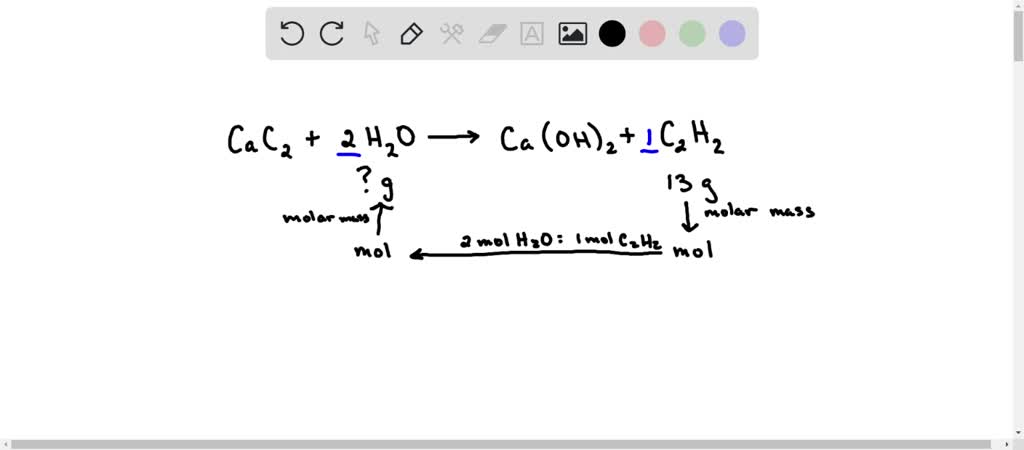
SOLVED: Calcium carbide (CaC2) reacts with water to produce acetylene (C2H2): CaC2 (s) + 2H2O (g) → Ca(OH)2 (s) + C2H2 (g) Production of 13 g of C2H2 (26.04 g/mol) requires consumption
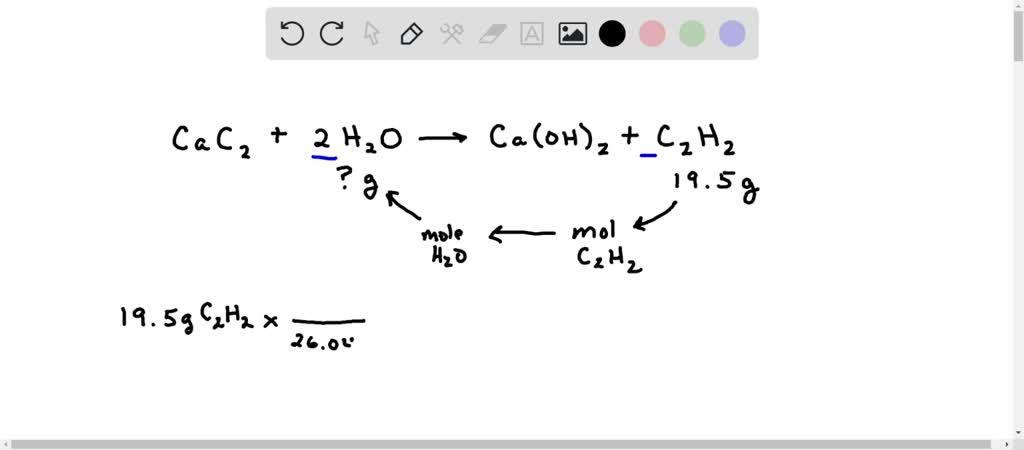
SOLVED: Calcium carbide (CaC2) reacts with water to form acetylene (C2H2): CaC2 (s) + 2 H2O (g) → Ca(OH)2 (s) + C2H2 (g) How many grams of water are required to produce







