Practice How many grams of H2O are produced by completely reacting 24 g of O2? 2 H2 + O2 2 H2O convert to moles convert to the desired molecule convert. - ppt download

The reaction, CO (g) + 3H2 (g) ⇌ CH4 (g) + H2O (g)is at equilibrium at 1300 K in a 1L flask..... - YouTube

Consider the following reaction: H2O(l)→H2O(g);ΔH1 = 44kJ 2CH3OH(l) + 3O2→ 4H2O(l) + 2CO2(g) ; ΔH2 = - 1453kJ What is the value of Δ H for the second reaction if water vapor

See: Calculate the amount of heat released when 27.0 g H2O is cooled from a liquid at 314 K to a solid at - Brainly.com

OneClass: 4) For the reaction H2(g) + CO2(g) H2O(g) + CO(g) at 700°C, Kp = 0.534. Calculate the numb...
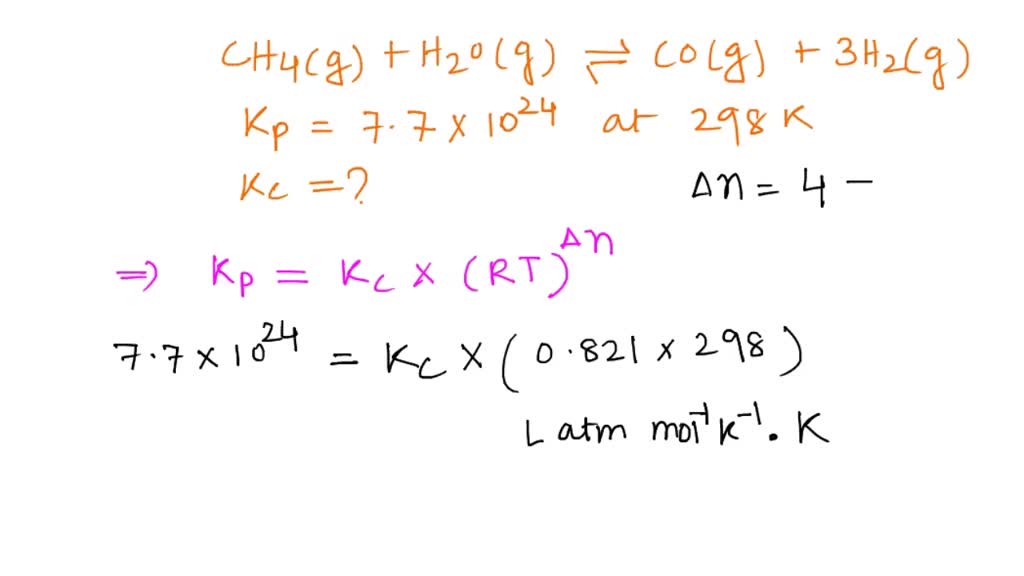
SOLVED: Consider the reaction below. H2O (g) + CH4 (g) <—> CO (g) + 3H2 (g) Kc = 4.7 at 1400 K What is Kp for this reaction at 1400 K? 6.2 x 104 4.7 8.2 x 10^8