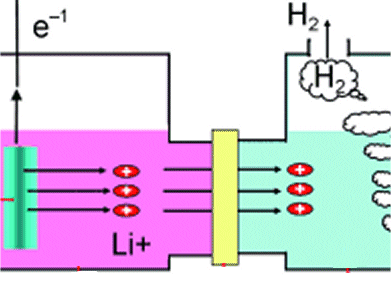
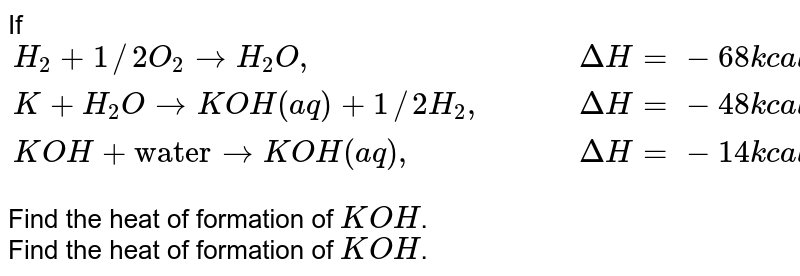n mole each of H2O, H2 and O2 are mixed at a suitable high temperature to attain the equilibrium 2H2O 2H2 + O2 . If y mole of H2O are dissociated and
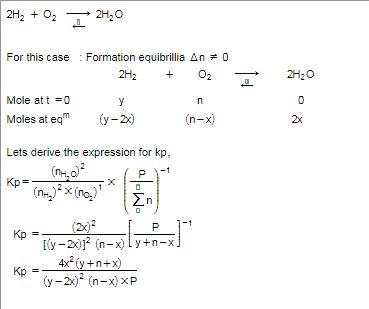
n mole each of h2o h2 o2 r tken in closed container at temperature t if y mole of h2 r disasssociated at equillibrium n equillibrium pressure is p the cgt66gee -Chemistry -
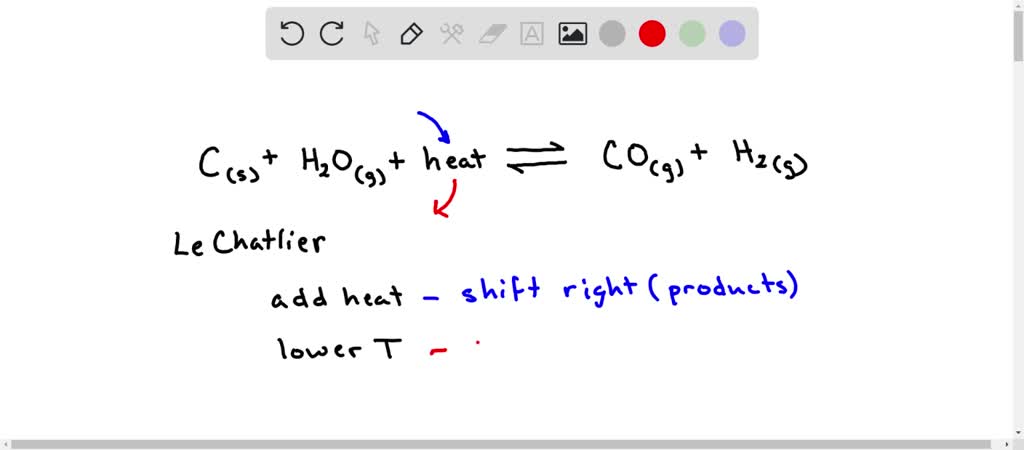
SOLVED: 5. When heated, carbon reacts with water to produce carbon monoxide and hydrogen. C( s ) + H2O(g) + heat ⇌CO(g) + H2(g) What effect does each of the following changes