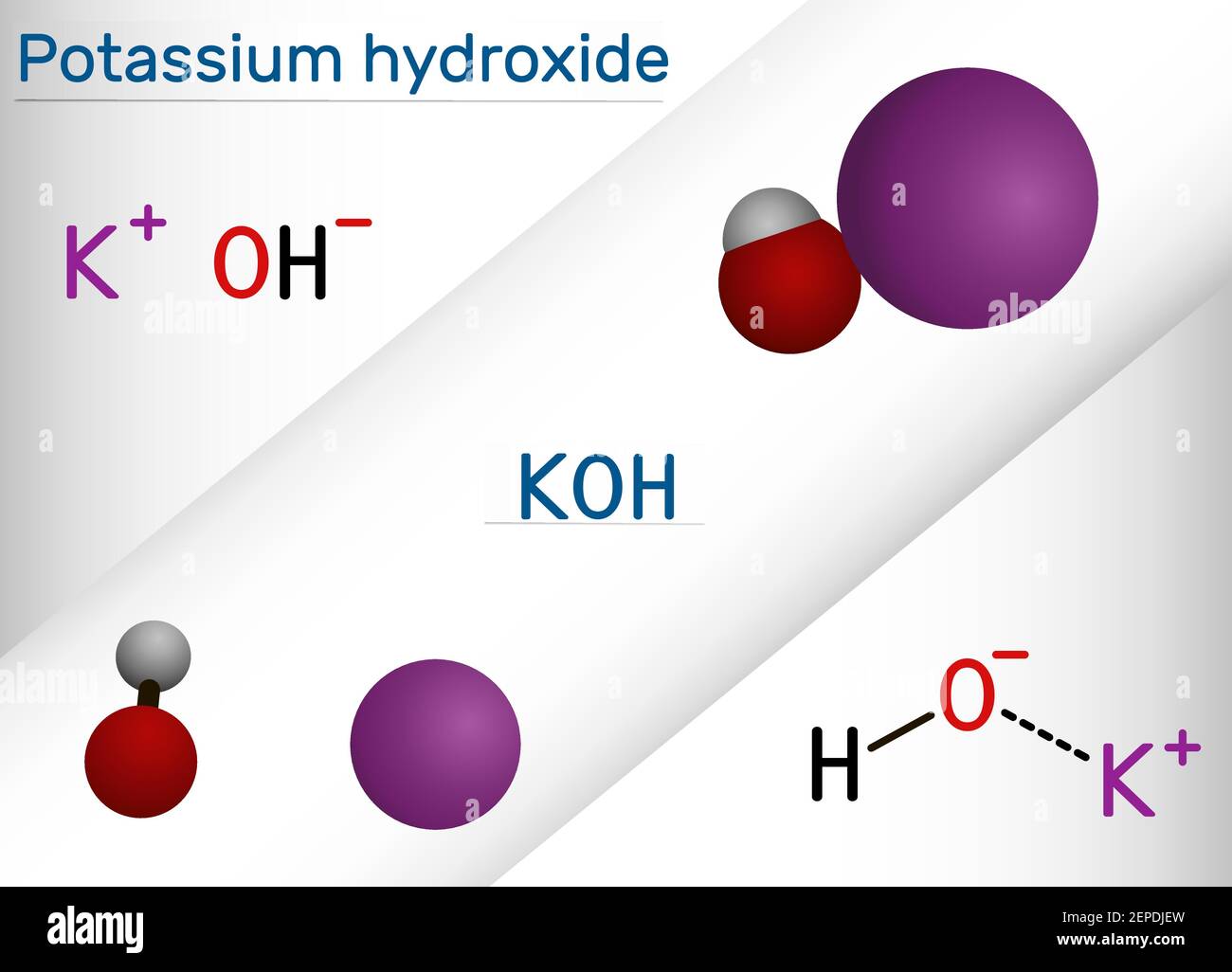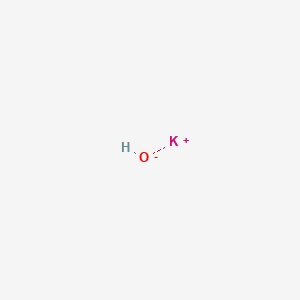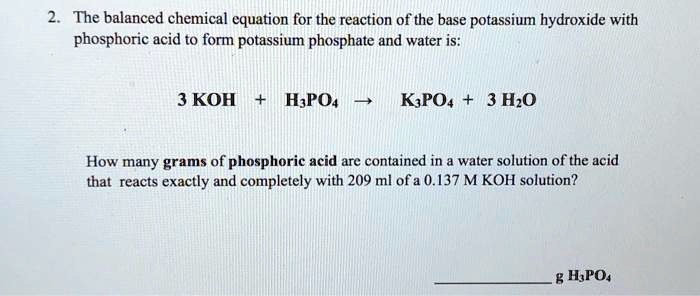
SOLVED: The balanced chemical equation for the reaction of the base potassium hydroxide with phosphoric acid to form potassium phosphate and water is: 3 KOH HPO4 KaPOs 3 HzO How many grams
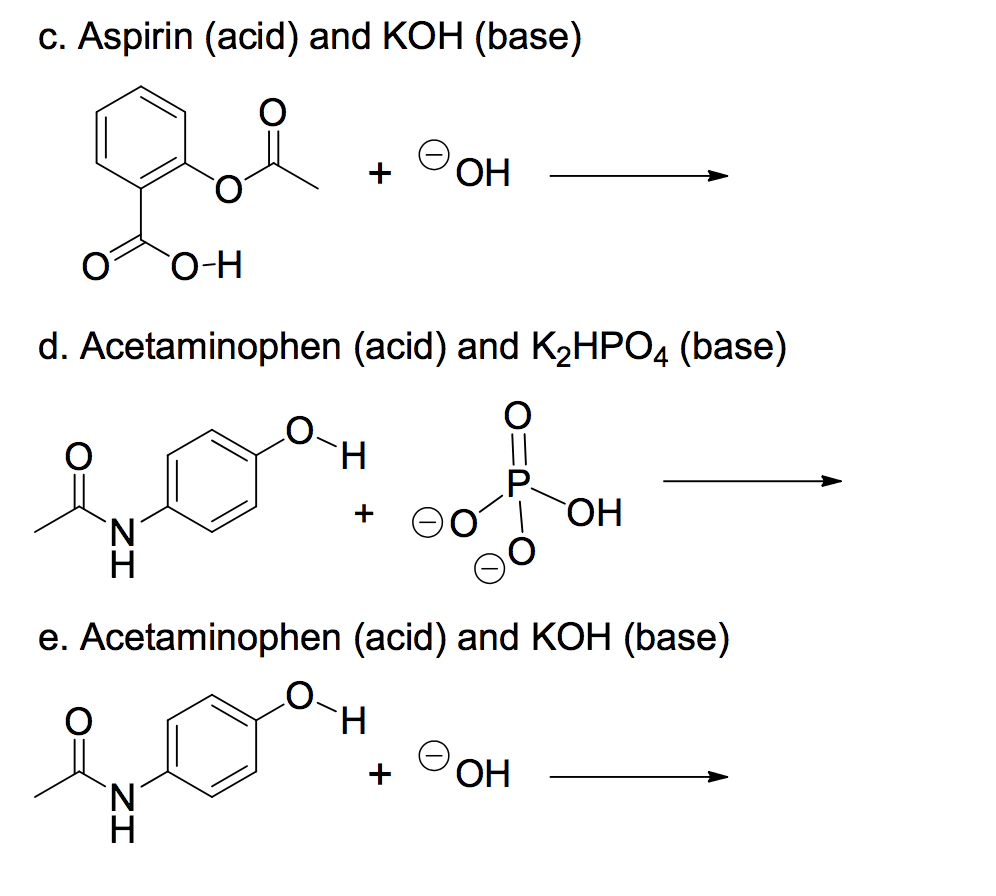
![Identify the reaction that occurs when the base KOH is added to the buffer solution. [{Image src='buffer_solution613505384566154416.jpg' alt='buffer solution' caption=''}] | Homework.Study.com Identify the reaction that occurs when the base KOH is added to the buffer solution. [{Image src='buffer_solution613505384566154416.jpg' alt='buffer solution' caption=''}] | Homework.Study.com](https://homework.study.com/cimages/multimages/16/buffer_solution613505384566154416.jpg)
Identify the reaction that occurs when the base KOH is added to the buffer solution. [{Image src='buffer_solution613505384566154416.jpg' alt='buffer solution' caption=''}] | Homework.Study.com