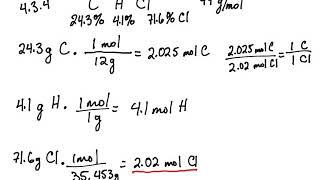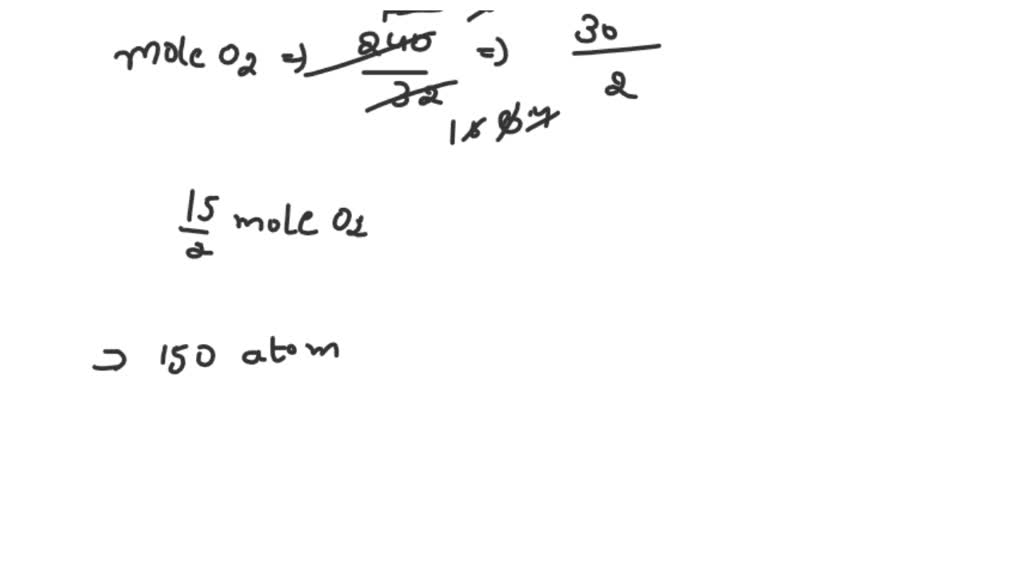
SOLVED: 1 mol of hydrocarbon P (contains C and H only) required 240 g of oxygen (O2) for complete combustion at standard state heat of combustion of P(l) is -3350 kJ/mol while
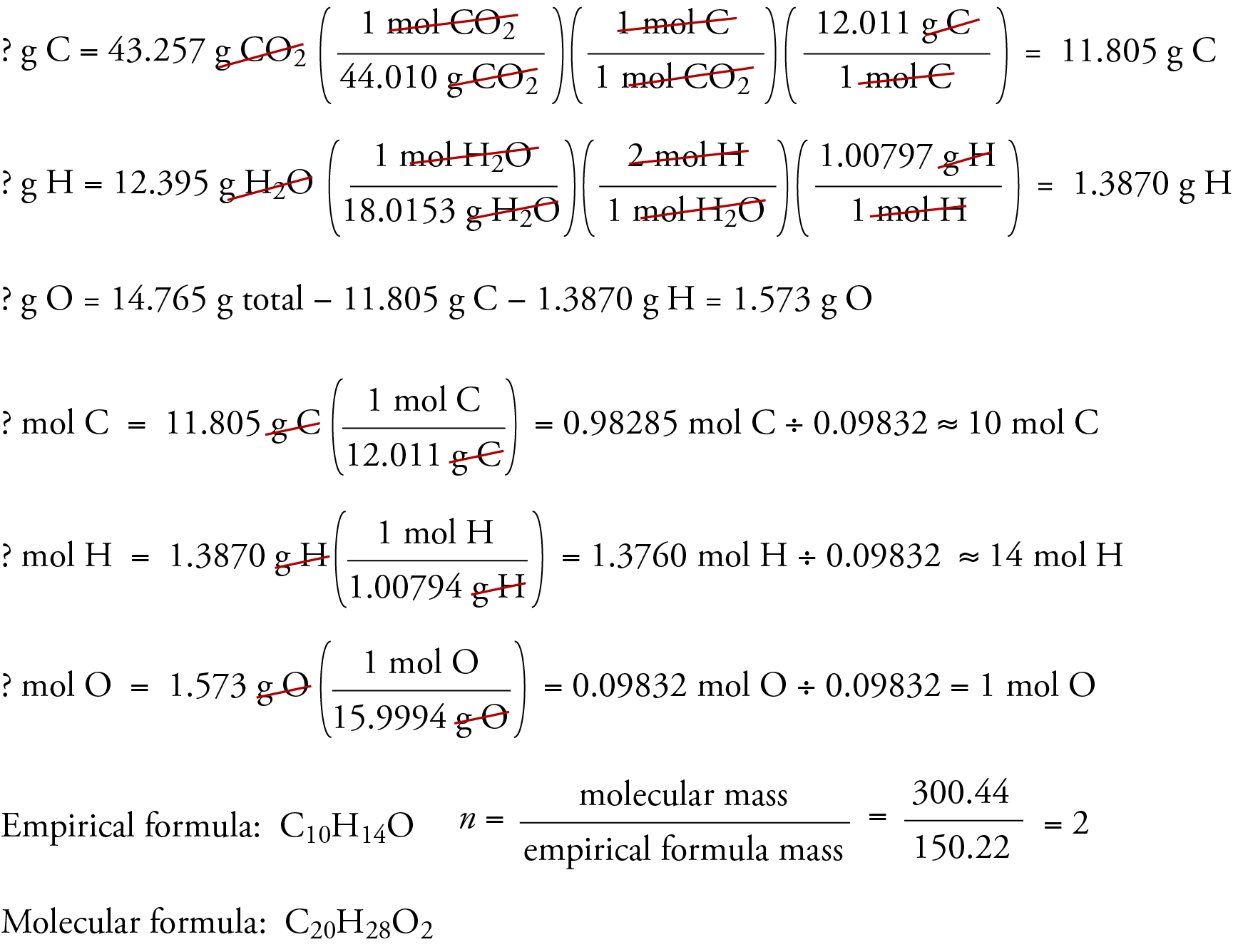
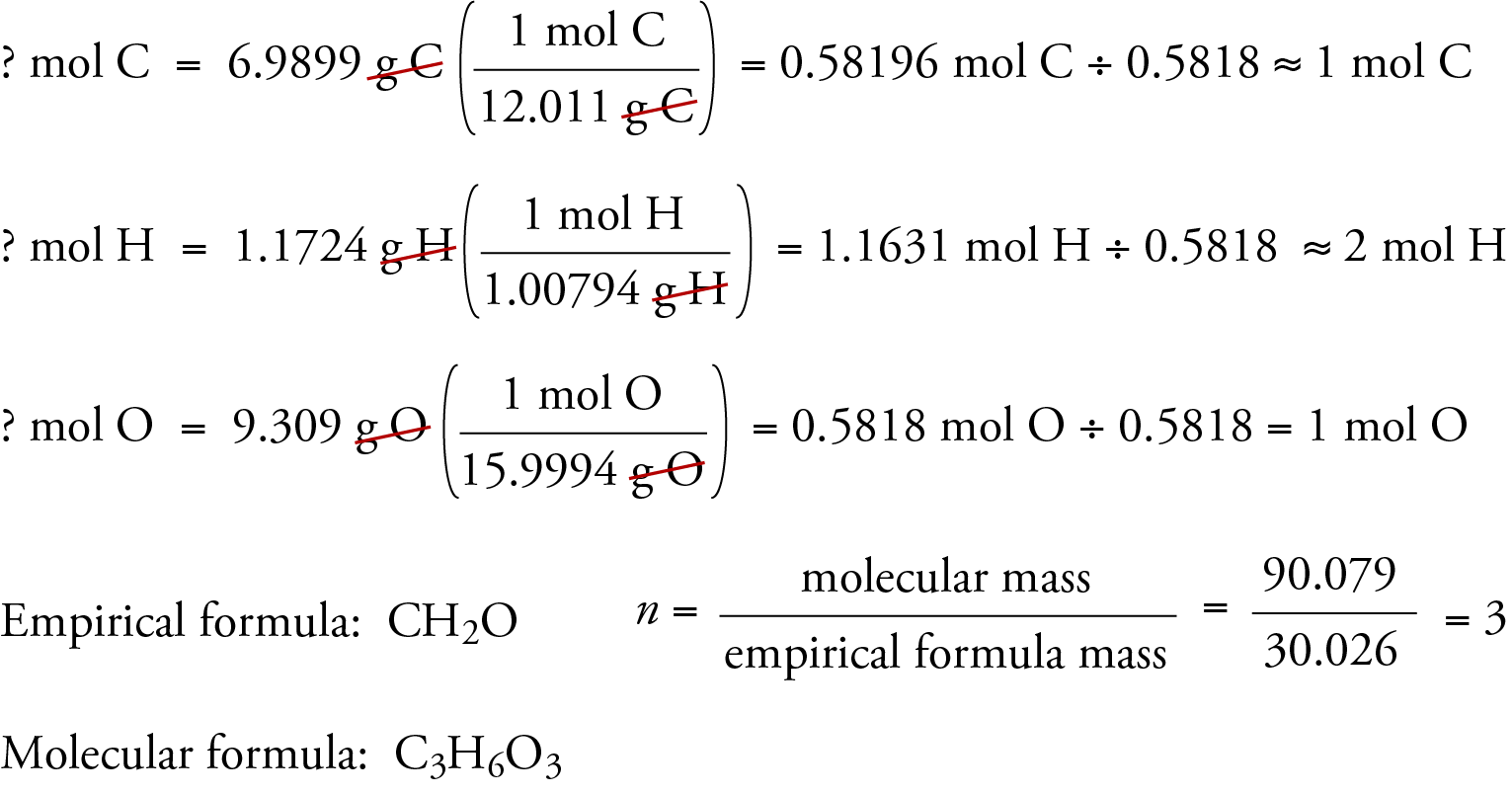
Empirical and molecular formulas for compounds that contain only carbon and hydrogen (C a H b ) or carbon, hydrogen, and oxygen (C a H b O c ) can be determined with a process called combustion analysis. The steps for this procedure are
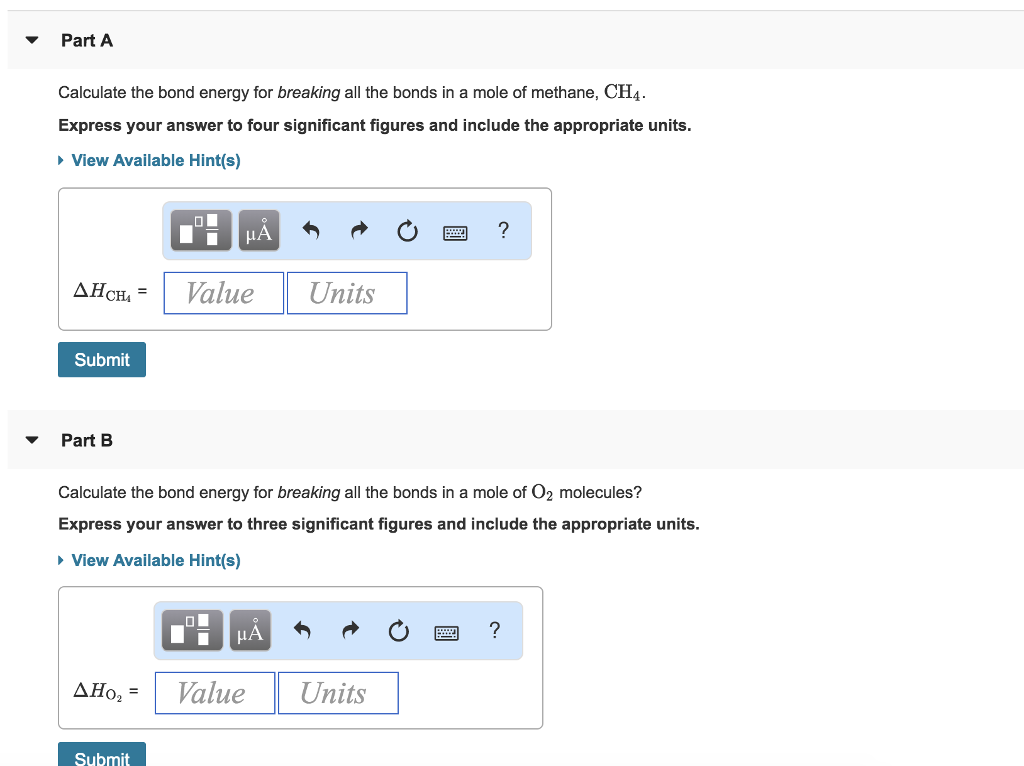
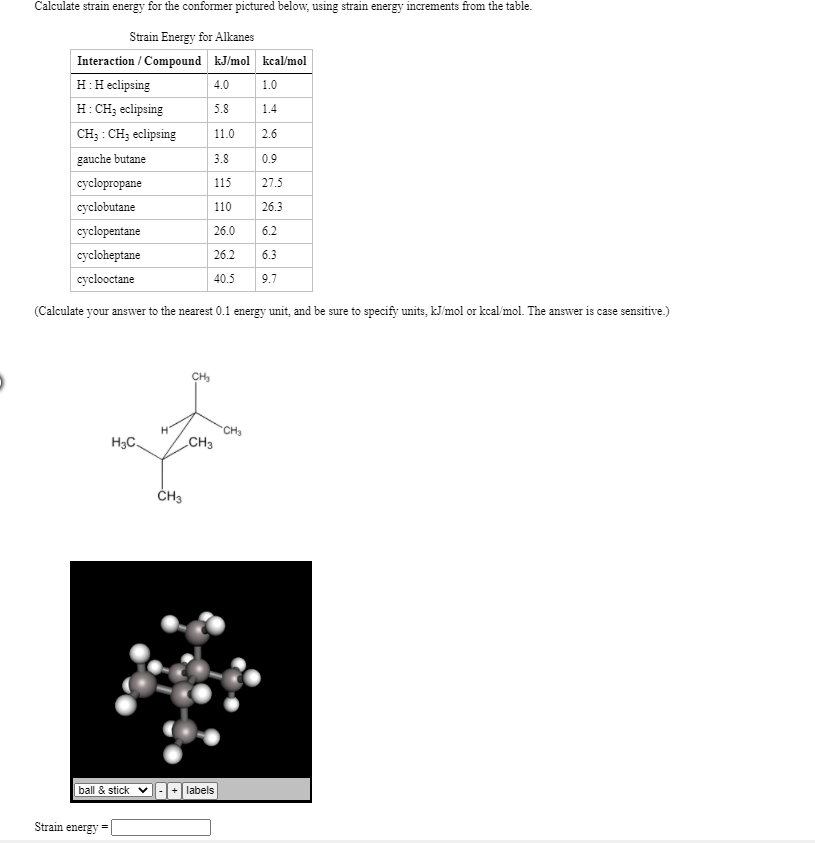
If △ H(c h)= 300 kJ/mol, △ H(atm) of C(s)= 500 kJ and △ H(h h) = 200 kJ/mol then the △ fH(CH4)will be

Chapter 11 : Matter Notes. Mole (mol) is equal to 6.02x10 23 The mole was named in honor of Amedeo Avogadro. He determined the volume of one mole of gas. - ppt download

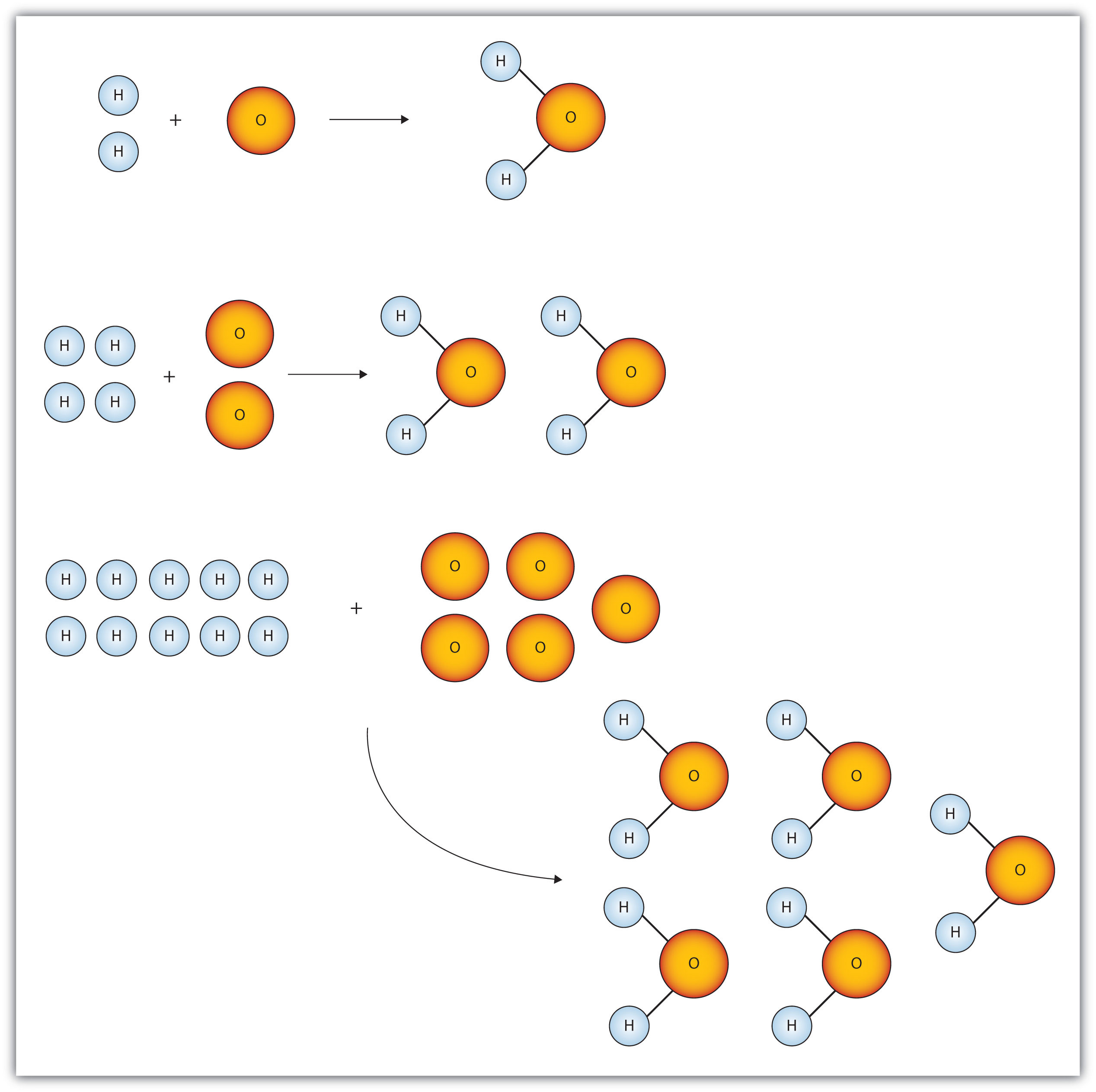
Mol ratio: coefficients of a balanced equation 2 H 2 + O 2 → 2 H 2 O 2 mol H 2 for every 1 mol O 2 In chemical calculations, mol ratios convert moles of. - ppt download