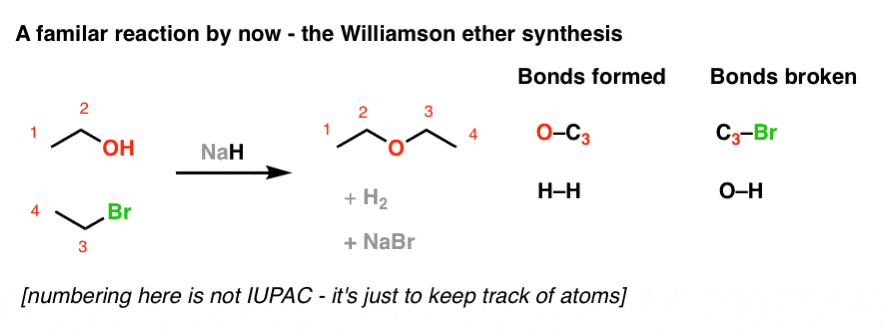
SOLVED: Sodium hydride is a source of hydride that can act as a (select all that apply) Na@ HO NaH = Strong base Strong nucleophile Weak base Weak nucleophile

When the halohydrin is treated with NaH, a product of molecular formula C_4H_8O is formed. Draw the structure of the product and indicate its stereochemistry. | Homework.Study.com

The hydride ion H^(-) is a stronger base than its hydroxide ion OH^(-). Which of the following reactions will occurs if sodium hydride (NaH) is dissolved in water ?
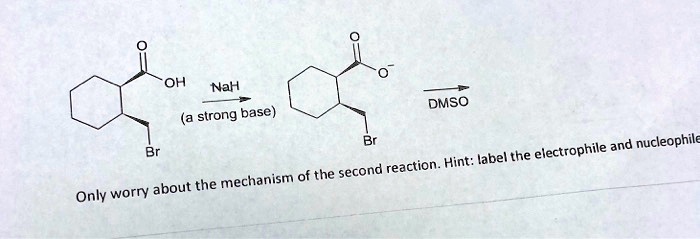
SOLVED: OH NaH DMSO strong base) and nucleophile Hint: label the electrophile second reaction mechanism of the Only worry about the

Commonly Used Hydride Reagents. Several forms of hydride (H-) find use in organic chemistry, including NaH, CaH 2, LiAlH 4, NaBH 4, and NaBH 3 CN (and. - ppt download

Complications from dual roles of sodium hydride as a base and as a reducing agent. - Abstract - Europe PMC