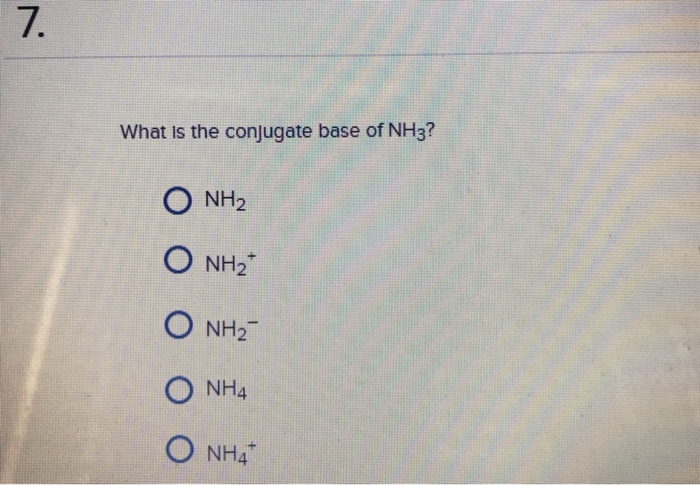
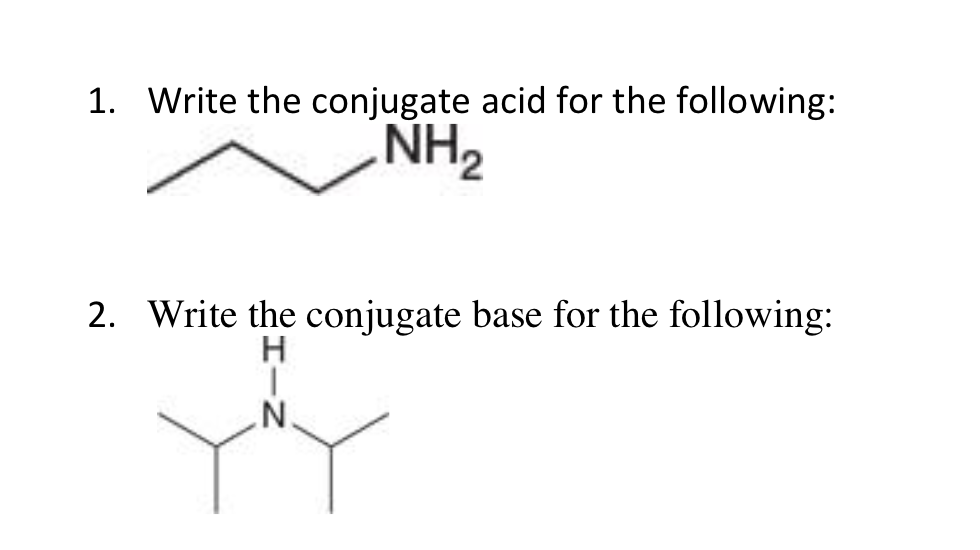
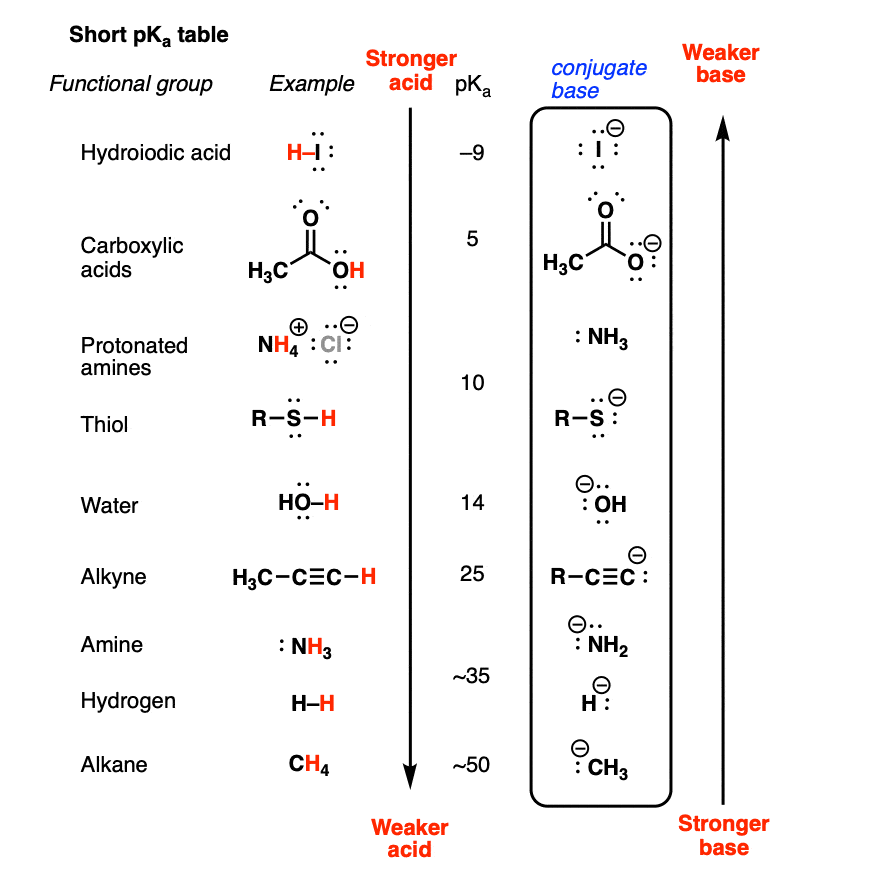
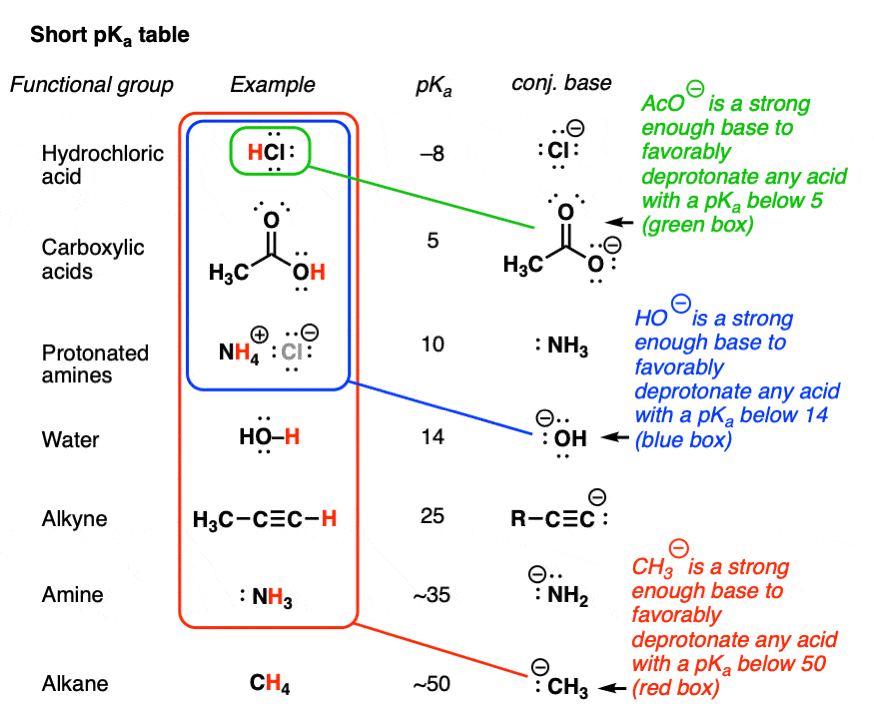
3.3: pKa of Organic Acids and Application of pKa to Predict Acid-Base Reaction Outcome - Chemistry LibreTexts

Schiff base adduct pathway for reaction between -NH2 group of arginine... | Download Scientific Diagram
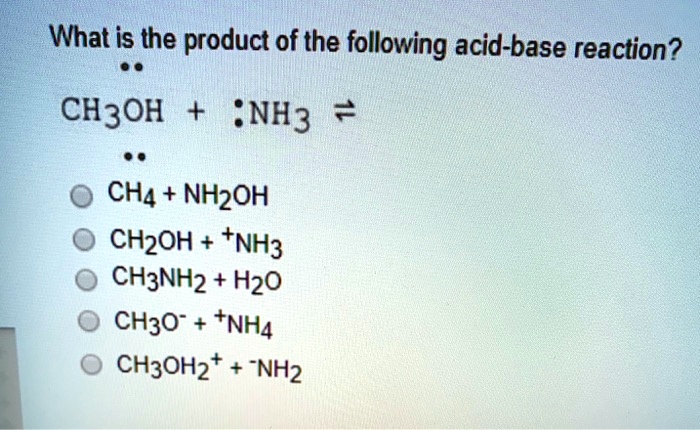
SOLVED: What is the product of the following acid-base reaction? CH3OH :NH3 2 CHA - NHZOH CH2OH +NH3 CH3NH2 Hzo CH3O" +NHA CH3OH2" NH2

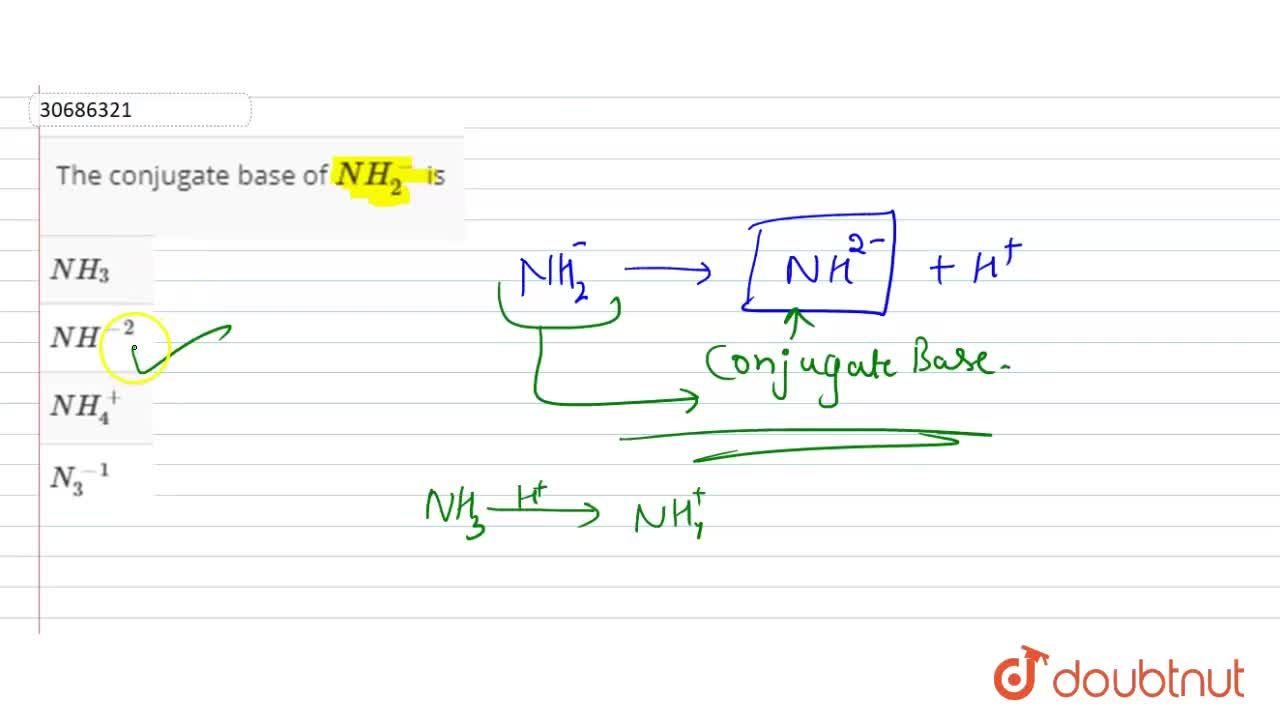
The correct decreasing order of basic strength of the following species is . H2O, NH3,OH^(-), NH2^(-)
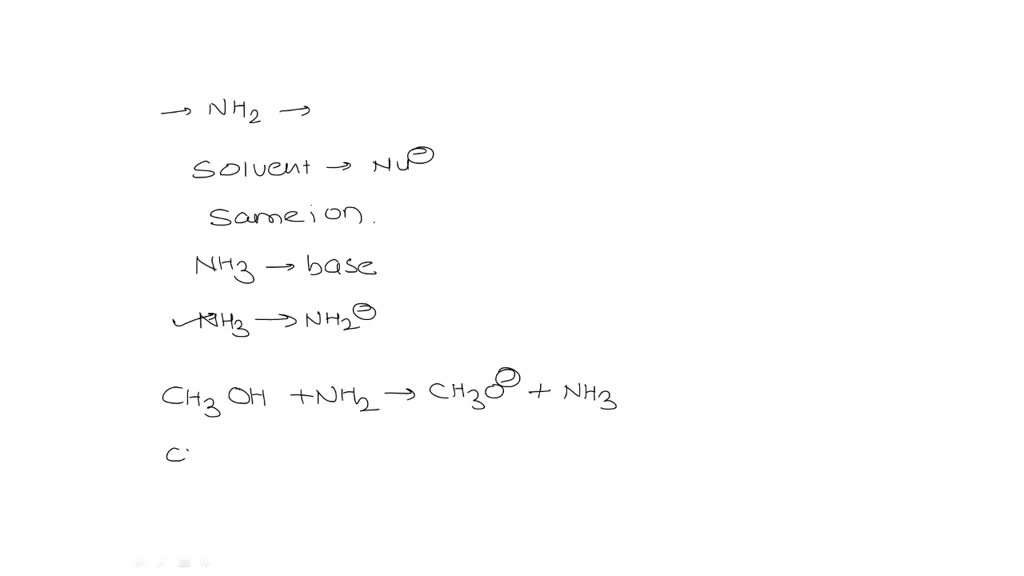
SOLVED: I already know the answer - please explain why I have no idea what this is talking about. The amide ion NH2- is a base which can only be used in